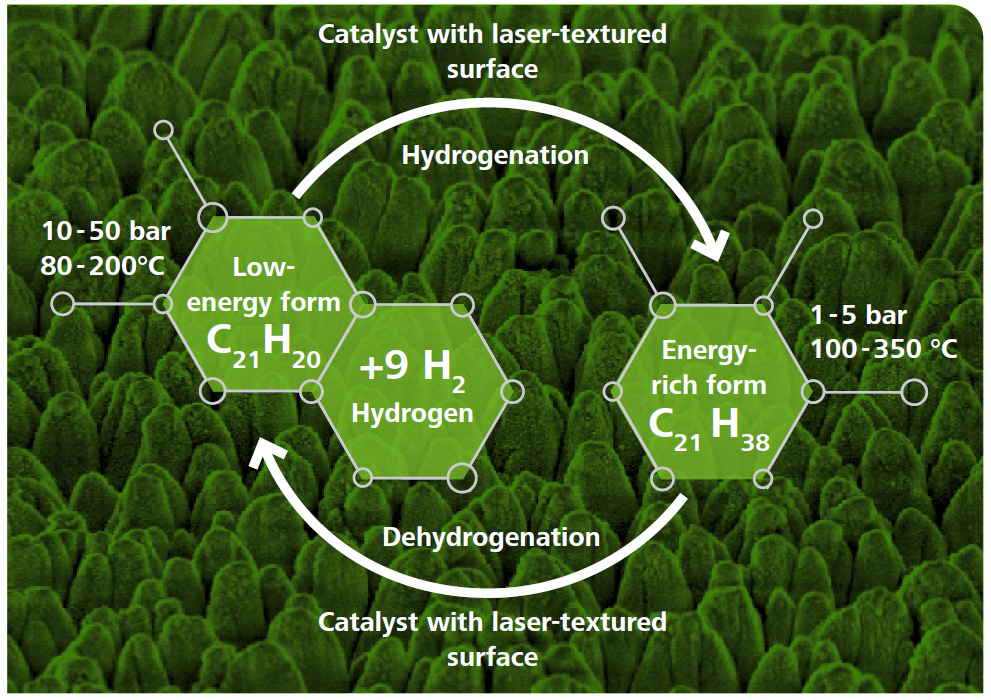
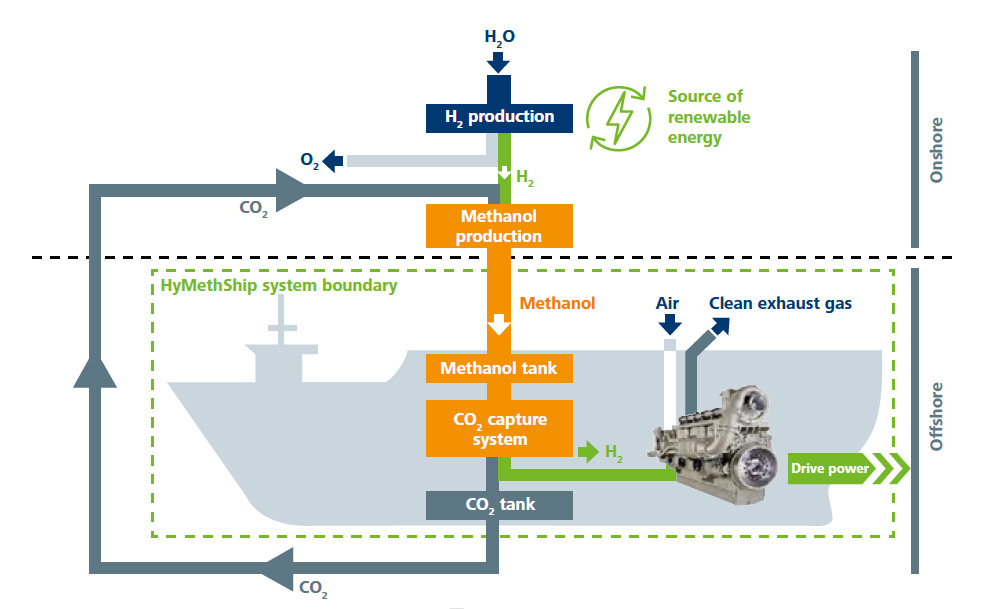
Is hydrogen also suitable for powering trucks, ships, trains and air-crafts?
Far less ideological is the question as to whether fuel cells or liquid fuels are better for propelling ships, trucks and aircrafts. In this instance, liquid fuels have clear advantages, thanks to their high energy density. After all, every ounce of weight counts, especially in aviation, where high performance over long operating times is a necessity.
In NAMOSYN, a project to develop synthetic fuels for sustainable transport, Fraunhofer ISE has teamed up with a host of partners. Tasks include the race to devise a cost-effective method of producing OMEs. This covers the entire value chain, starting with the feedstocks, CO2 and H2, and progressing to the development – including all the catalytic and separation processes – of a fuel that complies with current standards. In addition, the research consortium is investigating the in-engine use phase of OMEs, the compatibility of refueling infrastructure, the life cycle over the entire value chain and integration of these new fuels. “At Fraunhofer ISE, we’re looking at six alternative processes and evaluating them in terms of, for example, cost and carbon footprint,” says Dr. Achim Schaadt, head of department at Fraunhofer ISE. Vital to this work is a process simulation platform developed by the research team. This helps to identify the kind of process that would be required to produce a million metric tons of fuel a year. “There’s an interplay here between simulation and experimentation: we learn from the results achieved with small-scale plants and then feed these results into our simulation model,” explains Dr. Ouda Salem, head of the Power-to-Liquids group at Fraunhofer ISE. Another project partner is constructing a modular system with an output of 1 kilogram of OME an hour, while others are busy running engine tests. Incidentally, OMEs can be used not only as fuels but also as highly selective green solvents and CO2 sorbents.